
ATTENTION! TIME SENSITIVE!
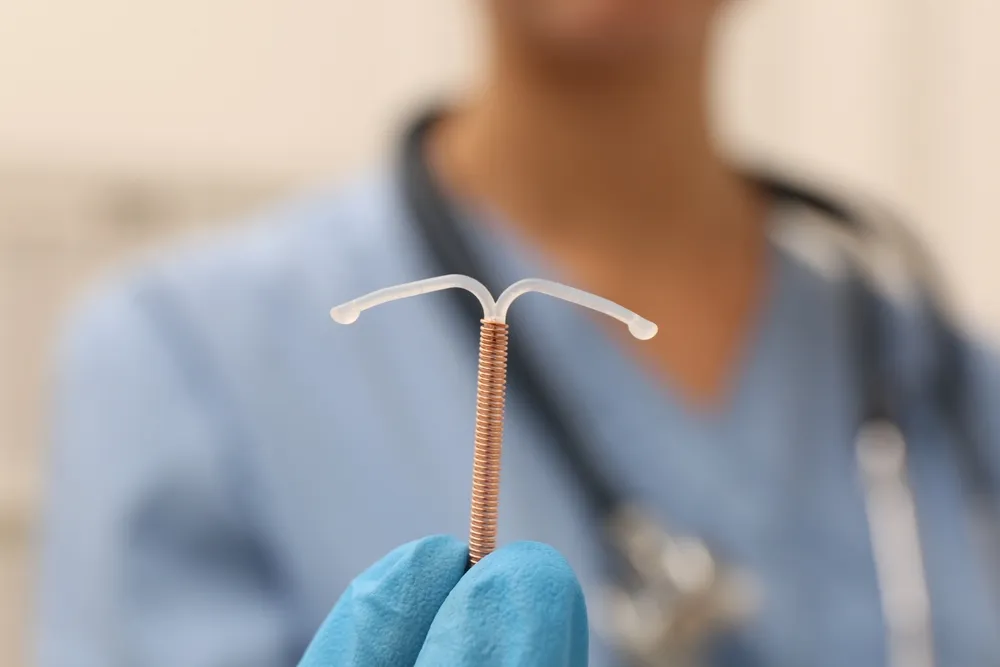

Paragard® IUD Breakages Cause Unexpected Trauma for Patients
When you chose Paragard as a birth control method, you likely trusted it would be safe, effective, and reliable for up to 10 years. Unfortunately, many people are now coming forward with their painful and life-altering experiences involving the Paragard® IUD breaking or fracturing. FDA studies have revealed that Paragard® IUDs can break within the body, causing trauma that in many cases requires invasive surgery. When the device fractures, it can lead to serious injuries, including perforation of the uterus, infections, scarring, and even potential loss of reproductive health. Disturbingly, the manufacturer recently admitted that the device can become “stuck” in the uterus, meaning surgical intervention may be required.
Unwarned Risks and Devastating Injuries
For many who have experienced complications, the real shock is that they were never warned of Paragard®’s tendency to break during removal. Lawsuits allege that the manufacturer failed to disclose the risk of fractures, leaving many unaware until they experienced serious injuries firsthand. For some, this breakage has resulted in fragments of the IUD being left inside the uterus, leading to infections, unplanned surgeries, and in some cases, harm to both mother and fetus during pregnancy. These complications are not just physical; they have left many individuals emotionally scarred and struggling with the impact of disrupted lives and damaged health.
Since 2010, the FDA has received more than 9,000 reports of Paragard® breakage. A 2022 analysis found that Paragard had nearly double the breakage rate of other IUDs approved in the U.S. Many individuals have reported excruciating pain during both the insertion and removal processes, yet they endured it in exchange for long-term, non-hormonal birth control. Tragically, the pain did not end there for some. The types of complications reported include:
Severe bleeding and cramping
Painful periods and intense abdominal pain
Unintended expulsion of the IUD
Irregular, foul-smelling discharge
Pain during sexual intercourse
Fragmented IUD pieces remaining embedded in the uterus
When these issues go untreated, they can lead to severe and life-altering consequences like infertility, ectopic pregnancy, miscarriage, pelvic inflammatory disease (PID), and even premature birth.
Undergoing Painful and Risky Surgeries
When a Paragard® IUD breaks, the process of removal can be invasive, painful, and unexpected. In some cases, the arms of the IUD remain lodged in the uterus, requiring extensive surgery to retrieve them. For many, this results in surgeries like hysteroscopy, laparoscopy, laparotomy, or even a full hysterectomy. Each of these procedures carries its own risks and, for some, can tragically affect their ability to conceive or carry a pregnancy in the future.
You Have Rights—And Options for Justice
Paragard® isn’t the first IUD to spark lawsuits. In recent years, thousands of people filed claims against Bayer for complications related to the Mirena® IUD, leading the company to pay over $12 million in settlements. Similarly, those affected by Paragard® have the right to hold the manufacturer accountable for failing to disclose the risks associated with their product.
At Litigation Notification, we believe that everyone should be fully informed of any risks associated with their birth control choices. We are dedicated to holding medical device manufacturers accountable for the pain and harm their products cause. If you or someone you care about has been harmed by the Paragard® IUD, our team is here to advocate for your rights and help you pursue the compensation you deserve for the damage done to your health and well-being.